For decades, the prevailing belief held that cancer arises from a gradual accumulation of genetic mutations in cells, ultimately leading to their malignant transformation. However, recent years have seen a revolutionary shift in this perspective. The tumor microenvironment (TME) has emerged as a dynamic and influential driver of cancer progression, prompting widespread recognition. Consequently, gaining a comprehensive understanding of the TME has become paramount in the quest for innovative cancer treatments.
At its core, the TME encompasses not only the tumor cells themselves but also an intricate web of microvessels, fibroblasts, immune cells, and more, all intricately connected to the tumor cells. The significance of delving into TME research cannot be overstated—it holds the key to unraveling the intricate processes underlying tumor formation, development, and metastasis. Moreover, it plays a crucial role in the diagnosis, prevention, and prognosis of tumors. Each case boasts a unique, complex, and ever-evolving TME, making the analysis of an individual's tumor microenvironment instrumental in tailoring personalized treatments and providing precise companion diagnostic information for targeted drug therapies.
While normal tissue microenvironments primarily maintain tissue homeostasis through intercellular interactions and the release of cytokines, the TME distinguishes itself through distinct cellular composition. Tumor cells, stromal cells, and immune cells intertwine to shape the TME's intricate landscape. Notably, tumor-associated macrophages (TAMs), regulatory T cells (Treg cells), and myeloid-derived suppressor cells (MDSCs) stand out as the primary immunosuppressive entities within the TME.
Tumor cells hold the power to recruit stromal cells from neighboring healthy tissues, subsequently enlisting them as key players in the microenvironment. These interstitial cells vary significantly across different tumor types and encompass vascular endothelium, fibroblasts, adipocytes, astrocytes, and more. Mesenchymal cells within the TME secrete an array of factors that participate in tumor angiogenesis, proliferation, invasion, and metastasis, playing critical roles in the progression of cancer.
Immune cells represent a pivotal component of the TME, with adaptive and innate immune cells shaping its landscape. Adaptive immune cells, including T cells, B cells, and natural killer cells (NK cells), are prevalent within the TME. Innate immune cells, such as macrophages, neutrophils, and dendritic cells, also abound. The interplay between immune cells and tumors classifies the immune microenvironment into two distinct categories: the anti-tumor immune microenvironment and the immune suppressive microenvironment. In the former, macrophages predominantly adopt an M1-type inflammatory phenotype, joining forces with NK and cytotoxic T cells to eliminate tumor cells. Neutrophils release reactive oxygen species (ROS), aiding Th1 T cells and B cells in secreting IFN-γ. Additionally, dendritic cells secrete TNF and IL-6, collectively maintaining an anti-tumor immune environment. Conversely, the immunosuppressive microenvironment features M2-type anti-inflammatory macrophages that collaborate with Treg cells and B cells to release IL-4, IL-10, and growth factors. These factors inhibit the activity of CD8+ T cells and dendritic cells, thus promoting tumor growth.
To comprehensively grasp the complexities of the tumor microenvironment, traditional immunohistochemistry (IHC) and next-generation sequencing (NGS) techniques prove insufficient. Enter multiplex immunohistochemistry (mIHC), a cutting-edge technology that has unveiled the intricate cellular interactions within complex tumor samples. mIHC empowers researchers to analyze the tumor microenvironment in greater depth, igniting a surge of momentum for clinicians, pathologists, and drug developers alike. Unlike standard IHC, which detects a single target protein, mIHC enables simultaneous detection of multiple targets in situ, making it an invaluable tool for studying intricate environments like the TME. By capturing tissue tumor markers, cell statuses, immune cell types, immune regulations, stromal cells, and other critical information, mIHC offers a holistic view of the tumor microenvironment.
The power of mIHC lies not only in its ability to provide high-dimensional data akin to single-cell omics but also in its capacity to analyze the spatial relationships between various elements within the tumor. This capacity paves the way for a detailed understanding of the tumor immune microenvironment, serving as the cornerstone for personalized medicine and companion diagnostics.
At Huabio, we provide meticulously verified Marker antibodies for tumor immune microenvironment research using mIHC. These reliable tools enable groundbreaking advancements in understanding the intricate interplay of tumor and immune cells. Together, we can unlock the mysteries of the tumor microenvironment and revolutionize cancer treatment.
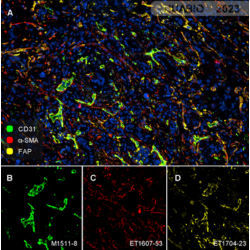
Human pancreatic carcinoma tissue
CD31 (M1511-8, green), α-SMA (ET1607-53, red) , FAP (ET1704-23, yellow)
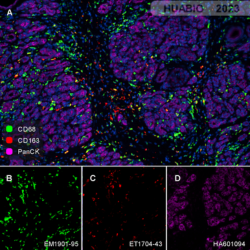
Human pancreatic carcinoma tissue
CD68 (EM1901-95, green), CD163 (ET1704-43, red) , PanCK (HA601094, violet)
References
1. doi:10.3389/fimmu.2019.01720
2.doi:10.1016/j.yexcr.2010.02.045
